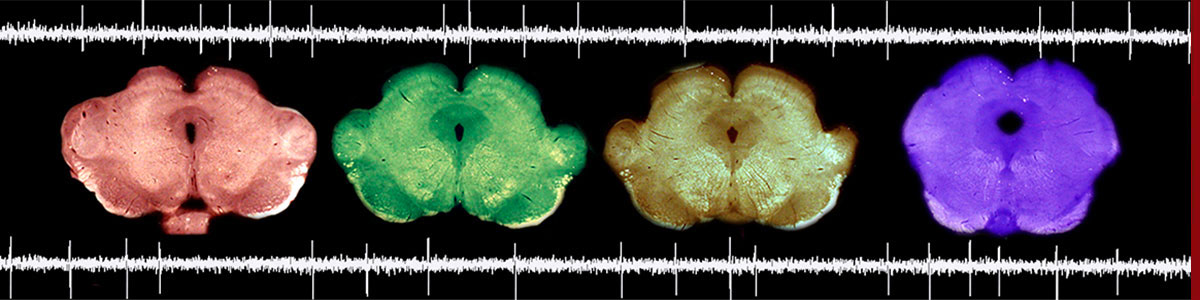
Transcriptional and epigenetic signatures of opiates
Pierre-Eric LUTZ

Presentation
 Opiates such as morphine and heroin have been both a benediction and a curse to mankind for centuries. While they remain the most efficient and prescribed painkillers in modern medicine, their chronic use also leads in some individuals to the emergence of addiction. Once hooked, affected individuals face a lifetime struggle to control, and hopefully abstain from, opiate consumption.
Opiates such as morphine and heroin have been both a benediction and a curse to mankind for centuries. While they remain the most efficient and prescribed painkillers in modern medicine, their chronic use also leads in some individuals to the emergence of addiction. Once hooked, affected individuals face a lifetime struggle to control, and hopefully abstain from, opiate consumption.
A major challenge is to understand how opiates can have such devastating and protracted effects on human behaviour. Our current projects seek to answer this important clinical and societal question, by exploring the hypothesis that epigenetic mechanisms may be critically implicated in these processes. Epigenetic mechanisms, such as DNA methylation and histone marks, correspond to chemical modifications that do not modify the DNA sequence, but control structural and functional organization of the genome. While they have been primarily implicated in cellular differentiation, accumulating data indicates that they remain active throughout life, even in post-mitotic neurons of the adult brain, where epigenetic reprogramming is progressively associated with a series of psychiatric phenotypes, including depression, addiction, and others.
In this context, our animal research program proposes to focus on the role of DNA methylation, a major epigenetic mark, in opiate effects.To do so, we combine genetic tools that we develop in the mouse, with next-generation sequencing methodologies and behavioural testing. Our goal is to produce comprehensive characterizations, in the entire genome, of DNA methylation, histone and gene expression adaptations that are triggered by opiates. Furthermore, we seek to characterize how these molecular substrates contribute to behavioural effects of opiates, focusing on their “dark side”: the potent aversive aspects of drug withdrawal, and the emergence of emotional dysfunction during prolonged abstinence from the drug.
Importantly, we are developing experimental strategies to conduct cell-type specific analyses of the neuronal population that expresses the mu opioid receptor, and represents the primary target of opiates. This endeavour is driven by the recognition that the mammalian brain is composed of multiple intermingled cell-types that exhibit huge transcriptomic and epigenetic diversity. This complexity severely hampers the detection of subtle cell-type specific processes that account for psychiatric symptoms, and go undetected otherwise. Implementing cell-type specific strategies is therefore critical, and our strategy has the potential to define the epigenetic blueprint of opiates with unprecedented resolution. In the long-term, we hope that this work will represent a main step forward for the understanding of opiate powerful properties.
See also: our research project in humans

Margot Diringer

Hanus Slavik

Yahia Hadj-Arab

Esther Colantonio
- Raoul Belzeaux, El Chérif Ibrahim, Eduardo Gascon (Institut de Neurosciences de la Timone, Marseille, France)
- Andrée Delahaye-Duriez (AP-HP, Neurodiderot Inserm UMR 1141, Paris, France)
- Bruno Etain, Cynthia Marie-Claire (Université Paris Descartes, INSERM UMR 1144, Paris, France)
- Laurence Lalanne, Emmanuel Darcq, Brigitte Kieffer (CRBS, INSERM U 1114, Strasbourg, France)
- Karine Merienne, Anne-Laurence Boutillier (LNCA, CNRS UMR 7364, Strasbourg, France)
- Anaïs Bardet (IGBMC, UMR 7104, Illkirch, France)
- Boris Chaumette (IPNP, INSERM U 1266, Paris, France)
- Gustavo Turecki (McGill Group for Suicide Studies, Douglas Hospital, McGill University, Montréal, Canada)
- Benoit Labonté (Institut CERVO, Université Laval, Québec, Canada)
Amazigh Mokhtari, PhD (actuellement ingénieur en sciences des données, Sanofi)
Ilona Bouvard, MSc (actuellement étudiante, Master Audiovisuel, journalisme et communication scientifiques, Paris)
- Genetically modified mice (knock-out and knock-in for opioid receptors and epigenetic enzymes)
- High-throughput genomics & epigenomics (RNA-Seq, Enzymatic Methylation sequencing, EPIC arrays, CUT&Tag)
- Quantitative PCR, immunohistochemistry, in situ hybridization
- In vivo fiber photometry (calcium, dopamine)
- Behavioral models of opioid responses: withdrawal-induced conditioned aversion; real-time place preference and aversion, emotional consequences of abstinence, psychomotor sensitization and tolerance
- Agence Nationale pour la Recherche (ANR)
- Institut pour la Recherche en Santé Publique / Institut National du Cancer (IReSP-INCa, AAP Addictions)
- Fondation Fyssen (subvention de recherche)
- Fondation de l’Avenir (recherche médicale appliquée)
- Université de Strasbourg (Idex Attractivité)
- Fondation pour la Recherche sur le Cerveau (FRC)
- Fondation pour la Recherche médicale (FRM équipe)
- American Foundation for Suicide Prevention (AFSP)