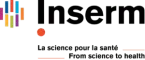Sex, painkillers and metabolism
Yannick GOUMON

Presentation
Two distinct projects are developed:
Role of the metabolism in the sexual dimorphism observed for the effects of morphine: analgesia and tolerance.
Morphine remains the gold standard to treat severe pain despite numerous side effects such as analgesic tolerance and addiction. It binds to Mu opioid receptors (MOR) expressed by many neurons, glial cells and immune cells. Within the central nervous system (CNS), and more specifically within pain control circuits, MORs activation promotes neuronal hyperpolarization hence the nociceptive message is inhibited.
Morphine is mainly metabolized into morphine-3-glucuronide by UDP-glucuronosyl transferases (UGTs) expressed in the liver. Metabolism is also observed in other organs and the CNS. Intriguingly, M3G is described to be pro-inflammatory and pro-nociceptive via a mechanism that is a matter of debate.
Among the side effects of morphine, morphine analgesic tolerance is characterized by the decrease in the analgesic effect over administration and thus the need to increase the doses to reach sufficient analgesia. Different mechanisms involving MORs receptors have been proposed to explain its development (e.g., phosphorylation of MORs, etc.). However, to date, no preventive or curative therapeutic strategies have been developed successfully.
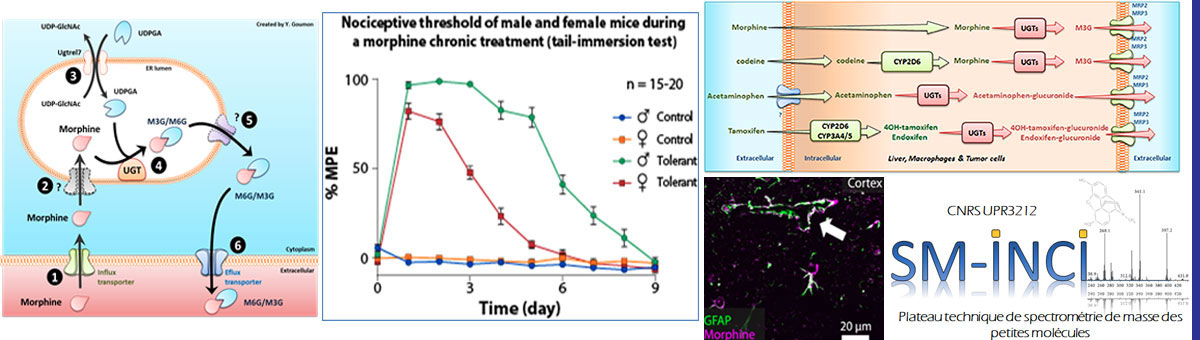
Interestingly, in rodents, the analgesic effect of morphine and its tolerance are strongly influenced by sex. Indeed, females require higher doses of morphine to achieve the same level of analgesia than males and become tolerant more quickly. Although the existing data in humans are more contrasted, these sex differences suggest inequalities in pain management in hospital settings.
Identifying the mechanisms underlying this sexual dimorphism could make possible to understand the development of morphine tolerance and designate new preventive or curative strategies. In addition, we aim to define the role of morphine (analgesia) metabolism to M3G (hyperalgesia) in the sex-related differences observed in opiates-induced analgesia. Deciphering sexual dimorphisms in pain control circuits will help to definitively consider sex as a variable for treating pain in the clinic.
Study of the impact of opioids and analgesics on breast cancer treatments.
In France, breast cancer is the most common cancer in women with nearly 55,000 cases detected per year and 11,000 deaths. The majority of breast cancers are estrogen receptor (ER) dependent. Thus, anti-tumor treatments target either ORs (e.g., tamoxifen) or the production of estrogen (e.g., anastrozole). After surgery, these chronic treatments are implemented over 5 years.
Tamoxifen and anastrozole are metabolized by cytochromes and UGTs. Unfortunately, cancer patients commonly develop chronic or neuropathic pain (> 50%, IASP data) following surgery and/or anti-tumor treatments (e.g., arthralgia). Pain relief is then achieved using chronic treatments with paracetamol, codeine or paracetamol-codeine. However, these painkillers share with tamoxifen and anastrozole the same metabolic pathways. These common degradation pathways suggest metabolic interactions which could be beneficial or harmful.
This project is based on a pilot study that has demonstrated that morphine dramatically affects the degradation of tamoxifen (Gabel & al. 2020, Front. Oncol.). It aims to study the impact of paracetamol and codeine on the metabolism of tamoxifen and anastrozole. The objective is to determine which analgesics can be used in patients treated for breast cancer to avoid any metabolic interactions, thus allowing better management of the patients. The objective is also to establish whether co-treatment can improve anti-tumour agents activity and develop new therapeutic strategies.
Technical approaches: behavior (nociceptive tests), surgeries, ip, it, icv injection, micro-dialysis, immunohistochemistry (epi-fluorescence & confocal), cell culture (lines and primary cultures), biochemistry (Western blot, ELISA), mass spectrometry (triple quad, isotope dilution method), electrophysiology and calcium imaging on brain slices and cell culture.
The team hosts the INCI mass spectrometry technical platform (MS-INCI; Technical Director V. Andry; Scientific Director Y. Goumon).