Opioid system, nociception and pain
Dominique MASSOTTE

 ⇨ Teams
⇨ Teams Presentation
 Neuropathic pain affects 7-8% of the population and has an annual economic cost of billion euros in Europe. This type of pain occurs as a direct consequence of a lesion or a disease affecting the somatosensory system. Sensory nerve injury, as well as peripheral and central maladaptive changes in neuronal network properties, lead to the appearance and development of neuropathic pain. The high prevalence and complexity of neuropathic pain syndromes represent a highly challenging medical need requiring effective therapeutic strategies. To date, first-line treatments include antidepressants or anticonvulsants which are sufficiently efficient in only 60% of the cases. For the other cases, opiates targeting mu opioid receptors are prescribed. However, repeated activation of these receptors induces side effects such as tolerance requiring increasing the dose to maintain the analgesic effect. To improve the efficacy of these treatments, it is therefore crucial to identify the alterations in endogenous opioid control that make it unable to prevent pain in neuropathic conditions and, that contribute to reducing the efficacy of drug treatments. The mechanisms leading to a maladaptive response of the endogenous opioid system in neuropathic conditions thus represent potential therapeutic targets. Therefore, our project aims at identifying these alterations to develop more effective therapeutic strategies. Furthermore, given the higher prevalence of neuropathic pain in women, a focus is also set on identifying sex-related differences in these mechanisms. To these aims, our team combines mouse models and analysis from molecular and cellular to integrated levels, mingling biochemical approaches, high-resolution fluorescence imaging, primary cultures, brain and spinal cord slices, electrophysiology, mass spectrometry and animal behavior.
Neuropathic pain affects 7-8% of the population and has an annual economic cost of billion euros in Europe. This type of pain occurs as a direct consequence of a lesion or a disease affecting the somatosensory system. Sensory nerve injury, as well as peripheral and central maladaptive changes in neuronal network properties, lead to the appearance and development of neuropathic pain. The high prevalence and complexity of neuropathic pain syndromes represent a highly challenging medical need requiring effective therapeutic strategies. To date, first-line treatments include antidepressants or anticonvulsants which are sufficiently efficient in only 60% of the cases. For the other cases, opiates targeting mu opioid receptors are prescribed. However, repeated activation of these receptors induces side effects such as tolerance requiring increasing the dose to maintain the analgesic effect. To improve the efficacy of these treatments, it is therefore crucial to identify the alterations in endogenous opioid control that make it unable to prevent pain in neuropathic conditions and, that contribute to reducing the efficacy of drug treatments. The mechanisms leading to a maladaptive response of the endogenous opioid system in neuropathic conditions thus represent potential therapeutic targets. Therefore, our project aims at identifying these alterations to develop more effective therapeutic strategies. Furthermore, given the higher prevalence of neuropathic pain in women, a focus is also set on identifying sex-related differences in these mechanisms. To these aims, our team combines mouse models and analysis from molecular and cellular to integrated levels, mingling biochemical approaches, high-resolution fluorescence imaging, primary cultures, brain and spinal cord slices, electrophysiology, mass spectrometry and animal behavior. > Modulation and plasticity of spinal inhibition
Perrine INQUIMBERT
> Functional dynamics of opioid receptors
Dominique MASSOTTE

Virginie Andry

Chantal Fitterer

Catherine Moreau

(Ph. D.)
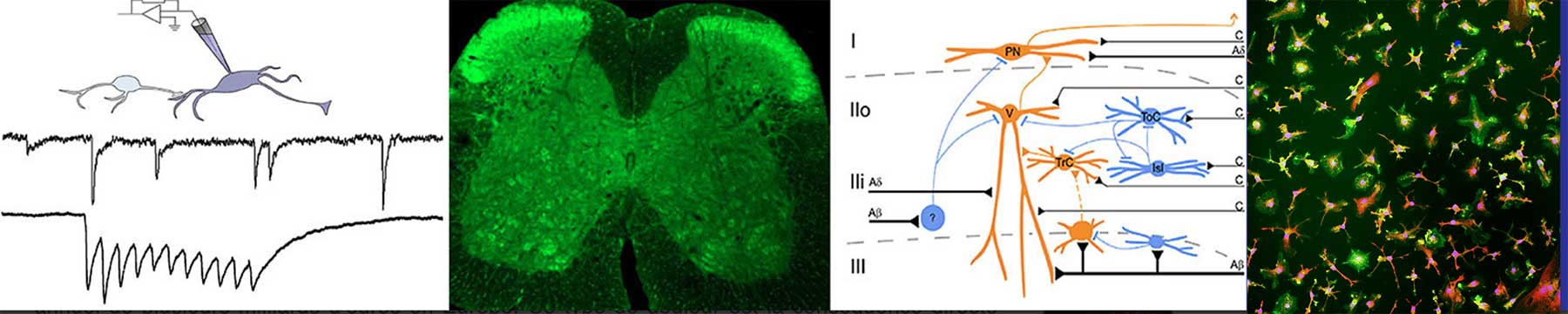
Nociceptive information, conveyed from the periphery by primary afferent fibers, is integrated and processed by a network of interneurons in the dorsal horn of the spinal cord. The excitation/inhibition balance in this network is critical to shape the nociceptive information. Short-term plasticity of inhibitory synapses within the lamina II, as a part of the nociceptive processing in the dorsal horn, could tune pain sensitivity. Through electrophysiological recordings on spinal cord slices, I am able to highlight this plasticity of inhibitory synapses depending on excitatory or inhibitory postsynaptic neurons. My goal is to examine whether the nociceptive thresholds in male and female mice can be correlated by the excitatory / inhibitory balance in the lamina II and its alteration during acute inflammatory condition.

(Ph. D.)
Neuropathic pain affects approximately 7 to 10% of the population. This type of pain is characterized by its chronicity and is associated with comorbid anxiety, depression, and cognitive deficits. To study the mechanisms underlying neuropathy, we focus on neurons expressing heteromers resulting from the association of mu and delta opioid receptors. In a mouse model of neuropathic pain, we use immunohistochemical detection, as well as a mass spectrometry approach to elucidate the brain networks, neuronal populations and neurotransmitters involved upon pharmacological activation of mu-delta heteromers.

(Ph. D.)
Neuropathy is associated with neuroinflammation, which leads to metabolic dysregulation. Morphine, used to relieve neuropathic pain, is less analgesic compared with its efficacy against acute pain. Our aim is to understand whether neuropathy influences morphine degradation in order to develop new strategies for effective analgesia for this type of pain.

Volodia Hovhannisyan
Cathenaut L, Schlichter R & Hugel S. Short-term plasticity in the spinal nociceptive system. Pain (2023), 164: 2411-2424.
Cathenaut L, Leonardon B, Kuster R, Inquimbert P, Schlichter R & Hugel S. Inhibitory interneurons with differential plasticities at their connections tune excitatory/inhibitory balance in the spinal nociceptive system. Pain (2022). 163(5):e675-e688.
Gabel F, Hovhannisyan V, Andry V, Goumon Y. Central metabolism as a potential origin of sex differences in morphine antinociception but not induction of antinociceptive tolerance in mice. Br J Pharmacol. 2023 Apr;180(7):843-861. doi: 10.1111/bph.15792. Epub 2022 Feb 8. PMID: 34986502.
Gaborit M. and Massotte D. (2023) “Therapeutic potential of opioid receptor heteromers in chronic pain and associated comorbidities” The British Journal of Pharmacology 180(7), 994-1013.