Presentation
Mood disorders are among the most commonly diagnosed disabling mental diseases. Despite the numerous pathophysiological hypotheses, elucidating the neurobiological basis of depression remains one of the foremost challenges in modern psychiatry.
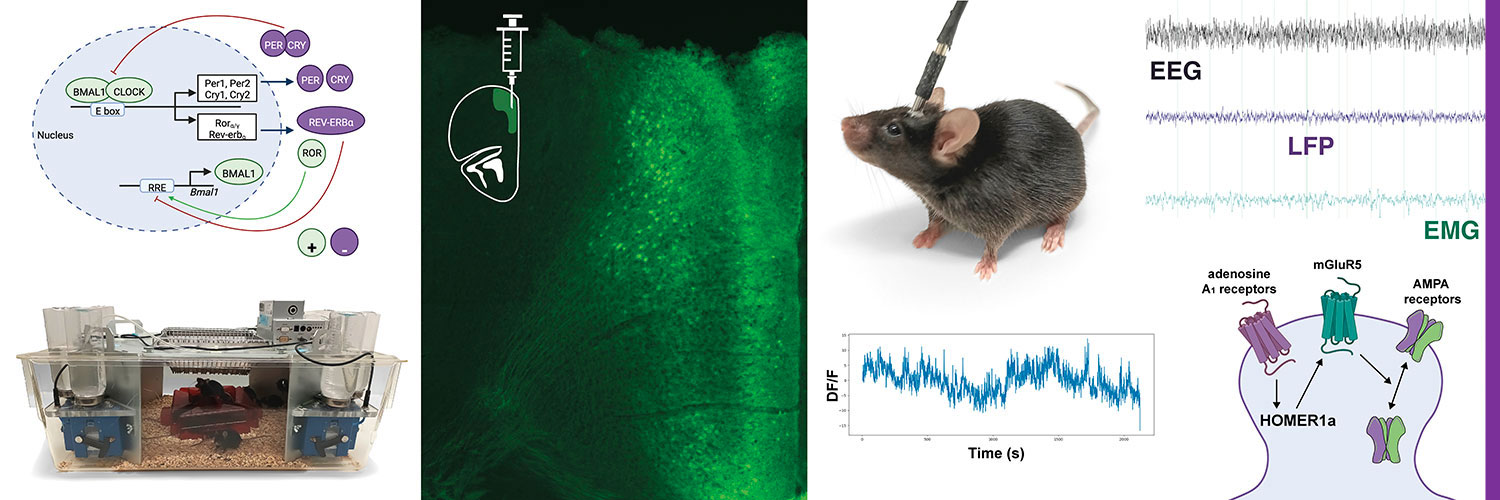
Disruptions of sleep and circadian rhythm are associated with the development of various psychiatric diseases. Chronotherapies, which include a variety of strategies that modulate biological clock and sleep-wake cycle, such as sleep deprivation and light therapy, are alternative treatments for mood disorders. Our previous work demonstrates that the rapid antidepressant effects of acute sleep deprivation depend on enhanced glutamatergic signaling. However, the involvement of the circadian clock in the antidepressant action and the pathophysiology of mood disorders is not yet well understood.
Using a combination of molecular biological methods, in vivo electrophysiology and complex behavioural analyses, we study the molecular and cellular mechanism of action of rapid antidepressant treatments and neurobiology of mood disorders. In particularly we aim:
- To investigate the role of the circadian clock in the pathophysiology and treatment of depression and bipolar disorder
- To characterize the crosstalk between circadian and sleep homeostatic mechanisms on glutamatergic signaling in mood regulation
- To study the impact of light in the antidepressant action of acute sleep deprivation

Tsvetan Serchov

Wilf Gardner (postdoctoral fellow)
- Prof. Dr. Claus Normann (Psychiatry, Uniklinik Freiburg)
- Prof. Dr. Bernd Fakler (Physiology, University Freiburg)
Genetically modified mouse models
Quantitative PCR, SDS-PAGE, Western blotting, Immunohystochemistry
Classical behavioral tests and complex automatic behavioral modeling and analyses via IntelliCage
In Vivo Electrophysiology: LFP and EEG (sleep) recordings
Van Calker D, Serchov T*. (2021) The “Missing Heritability”-Problem in Psychiatry: Is the interaction of Genetics, Epigenetics and Transposable Elements a potential solution? Neurosci Biobehav Rev. S0149-7634 (21) 00134-2. doi: 10.1016/j.neubiorev. 2021.03.019
Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, Biojone C, Cannarozzo C, Sahu MP, Kaurinkoski K, Brunello CA, Steinzeig A, Winkel F, Patil S, Vestring S, Serchov T, Diniz CRAF, Laukkanen L, Cardon I, Antila H, Rog T, Piepponen TP, Bramham CR, Normann C, Lauri SE, Saarma M, Vattulainen I, Castrén E. (2021) Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 184(5):1299-1313.e19. doi: 10.1016/j.cell.2021.01.034.
Sun L, Verkaik-Schakel RK, Biber K, Plösch T, Serchov T* (2021) Antidepressant treatment is associated with epigenetic alterations of Homer1 promoter in a mouse model of chronic depression J Affect Disord. 279:501-509, doi: 10.1016/j.jad.2020.10.040.
Serchov T*, Schwarz I, Theiss A, Sun L, Holz A, Döbrössy MD, Schwarz MK, Normann C, Biber K, van Calker D. (2019) Enhanced adenosine A1 receptor and Homer1a expression in hippocampus modulates the resilience to stress-induced depression-like behavior. Neuropharmacology. 107834. doi: 10.1016/j.neuropharm.2019.
Holz A, Mülsch F, Schwarz MK, Hollmann M, Döbrössy MD, Coenen VA, Bartos M, Normann C, Biber K, van Calker D, Serchov T*. (2019) Enhanced mGlu5 Signaling in Excitatory Neurons Promotes Rapid Antidepressant Effects via AMPA Receptor Activation. Neuron. pii: S0896-6273(19)30637-3. doi: 10.1016/j.neuron.2019.07.011.
Van Calker D, Biber K, Domschke K, Serchov T*. (2019) The Role of Adenosine Receptors in Mood and Anxiety Disorders. J Neurochem. doi: 10.1111/jnc.14841.
Serchov T, Clement HW, Schwarz MK, Iasevoli F, Tosh DK, Idzko M, Jacobson KA, de Bartolomeis A, Normann C, Biber K, van Calker D. (2015) Increased Signaling via Adenosine A1 Receptors, Sleep Deprivation, Imipramine, and Ketamine Inhibit Depressive-like Behavior via Induction of Homer1a. Neuron. 87(3):549-62. doi: 10.1016/j.neuron.2015.07.010.