Presentation
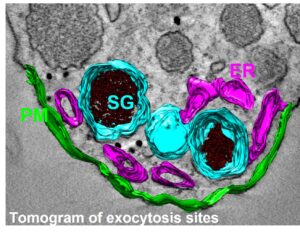
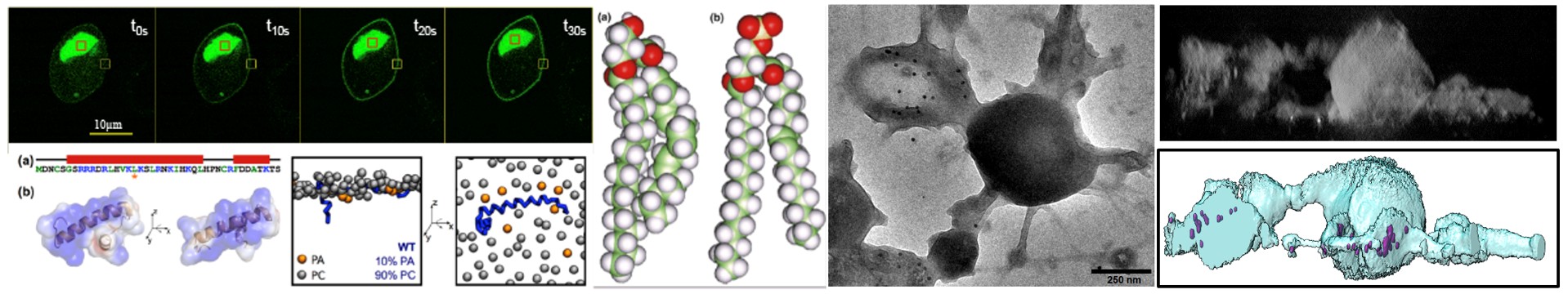
Regulated exocytosis is a complex process that enables the release of informative molecules stored in dedicated vesicles to enable cell-to-cell communication in multicellular organisms. This process begins with vesicle genesis, continues with vesicle transport, culminating in vesicle docking to the plasma membrane and fusion in response to elevated cytosolic calcium, allowing the release of vesicle contents outside the cell, and ends with the recycling of granular components by compensatory endocytosis. Numerous proteins playing a key role in these processes have been identified in recent decades. Although this process involves specific membrane compartments, the role of lipids, major constituents of membranes, and their dynamics between these compartments, remain much less understood.
The aim of this project is to understand the role of contact sites between the endoplasmic reticulum, secretory granules and the plasma membrane in regulating the cycle of exocytosis and endocytosis. In particular, we are studying the dynamics of calcium in the vicinity of exocytosis sites, and the contribution of different forms of phosphatidic acid (PA) during the various stages of exocytosis and their dynamics within these inter-organelle contact sites.
To answer these questions, we are using a molecular toolbox dedicated to the study of PA, based on optogenetic approaches and the use of new forms of PA synthesis that can be activated on demand on the basis of click chemistry.

Sebahat Ozkan
Pr Maité Montero : DC2N laboratory, Rouen
Pr Pierre-Yves Renard : CARMEN laboratory, Rouen
Dr Johann Böhm : IGBMC, Strasbourg
Dr Guillaume Drin : IPMC, Valbonne
Dr Oussama El Far : UNIS Laboratory, Marseille
Dr Franck Delavoie : University of Toulouse III, Toulouse
– Gabel M, Delavoie F, Demais V, Royer C, Bailly Y, Vitale N, Bader MF, Chasserot-Golaz S. Annexin A2-dependent actin bundling promotes secretory granule docking to the plasma membrane and exocytosis.
J Cell Biol 2015 Aug 31;210(5):785-800
– Kassas N, Tanguy E, Thahouly T, Fouillen L, Heintz D, Chasserot-Golaz S, Bader MF, Grant NJ, Vitale N. Comparative characterization of phosphatidic acid sensors and their localization during frustrated phagocytosis. J Biol Chem 2017, 292: 4266-4279.
– Tanguy E, Costé de Bagneaux P, Kassas N, Ammar MR, Wang Q, Haeberlé AM, Raherindratsara J, Fouillen L, Renard PY, Montero-Hadjadje M, Chasserot-Golaz S, Ory S, Gasman S, Bader MF and Vitale N. Mono- and poly-unsaturated phosphatidic acid regulate distinct steps of regulated exocytosis in neuroendocrine cells. Cell Reports 2020, 32(7):108026.
– Schlichter A, Wolf A, Ferrand T, Cocq A, Riachy L, Vertueux S, Beauvais B, Courvalet M, Henry PJ, Tanguy E, Gonzales L, Ferlet R, Laguerre F, Decraene C, Pellissier A, Sebban M, Sabot C, Jeandel L, Cianférani S, Strub JM, Bénard M, Flon V, Peulon-Agasse V, Cardinael P, Ory S, Gasman S, Renard PY, Montero-Hadjadje M, Vitale N, Balieu S. Designing New Natural-Mimetic Phosphatidic Acid: A Versatile and Innovative Synthetic Strategy for Glycerophospholipid Research. Angew Chem Int Ed Engl 2025 Jul 8:e202510412.
CNRS: Quand les lipides révèlent leurs secrets
Actualités INSERM: Le rôle de l’acide phosphatidique dans la sécrétion hormonale par les cellules neuroendocrines
Avanti Polar Lipids Research Spotlight: Mono- and Poly-unsaturated Phosphatidic Acid Regulates Distinct Steps of Regulated Exocytoses in Neuroendocrine Cells