Neuroendocrine mechanisms regulating seasonal functions
Valérie Simonneaux, Paul Klosen, Aurélia Ces, Vincent Joseph Poirel, Vebjorn Melum, Louise Sicot

Presentation
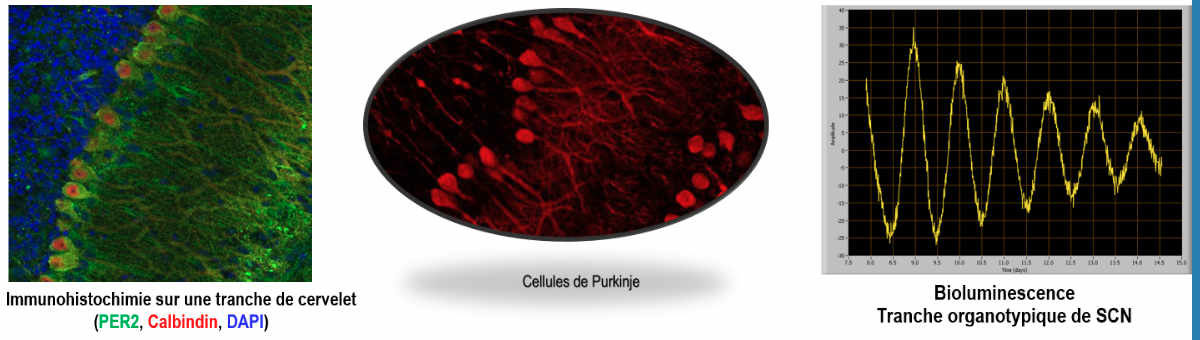
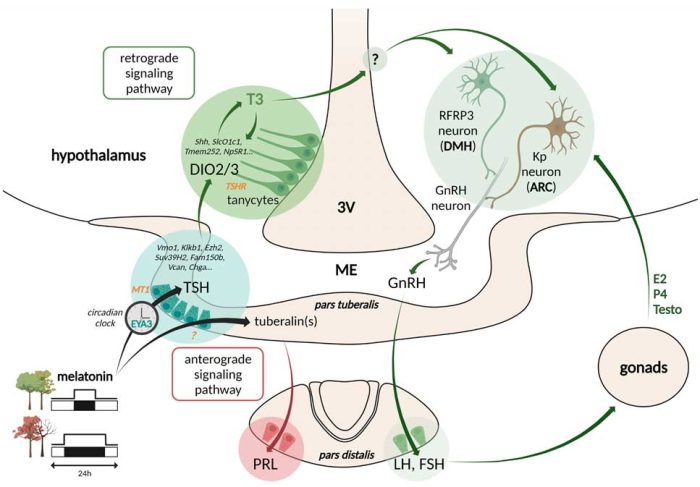
The seasonal change in the nocturnal secretion of the pineal hormone melatonin is decoded into a summer/long photoperiod increase in TSH (thyroid stimulating hormone) synthesis by the pars tuberalis of the hypophysis. TSH in turn increases the capacity of the tanycytes (radial glial cells located at the basal part of the 3rd ventricle) to convert T4 into the bioactive T3 in the neighboring hypothalamus (Dardente & Simonneaux, Front NeuroEndo, 2022 for review).
Our current objective is to test the hypothesis that tanycytes and specific hypothalamic neuronal populations are critical to adapt long term biological activities such as reproduction, metabolism, hibernation, and neurogliogenesis with the seasons.
Reproduction: The hypothalamic peptide (Arg)(Phe) related peptide 3 (RFRP3), known the regulate GnRH neuron activity, is strongly inhibited in short days/winter conditions in all seasonal mammals. We are testing the hypothesis of a pivotal role of RFRP3 neurons in the melatonin-driven adaptation of reproduction with the seasons in collaboration with Dr H. Dardente (INRA, Nouzilly; ANR GMO-Phen). In both Syrian hamster and sheep, we are developing the Crispr-Cas9 technology for unconventional to evaluate the effect of RFRP3 knock-out on seasonal reproduction, and we are comparing the photoperiodic change in the RFRP3 neuron transcriptome.
Hibernation: Syrian hamsters kept in short photoperiod at low temperature is able to hibernate. We are elucidating the neuroendocrine pathways involved in the onset/off set of hibernation cycles in collaboration with D. Hazlerigg and S. Wood from the Artic University of Tromso (Norway; INSB Diverity ion Biological Mechnisms fundings). Preliminary data show that TSH, infused icv via programmable refillable osmotic minipumps, prevent hypothermic bouts. Further, in specific hypothalamic areas, cells yet to be identified are activated at different stages of the hibernation cycle.
Seasonal neurogliogenesis: Seasonal adaptation involves large changes in hypothalamic cell plasticity and gene expression. Transfert in short photoperiod is associated with increased hypothalamic neuro-gliogeneis. We are investigating the seasonal signals regulating this short photoperiod-induced cell proliferation, the fate of the proliferative cells, and the putative role of the short photoperiod-induced genesis of these new hypothalamic cells in seasonal functions.
Seasonal maternal programming: Maternal melatonin is able to program offspring’s development, as hamsters born from a maternal gestation under summer/long photoperiod have a faster metabolic and reproductive development than hamsters born from a maternal gestation under winter/short photoperiod. In collaboration with S. Wood and D. Hazlerigg from the Artic University of Tromso (Norway), we are investigating the role tanaycyte plasticity in this melatonin-driven seasonal maternal programming.
Melum VJ, Sáenz de Miera C, Markussen FAF, Cázarez-Márquez F, Jaeger C, Sandve SR, Simonneaux V, Hazlerigg DG, Wood SH. Hypothalamic tanycytes as mediators of maternally programmed seasonal plasticity. Curr Biol. 2024 :S0960-9822(23)01742-6. doi: 10.1016/j.cub.2023.12.042
Dardente H, Simonneaux V. GnRH and the photoperiodic control of seasonal reproduction: Delegating the task to kisspeptin and RFRP-3. J Neuroendocrinol. 2022 34(5):e13124. doi: 10.1111/jne.13124
Sáenz de Miera C, Bothorel B, Jaeger C, Simonneaux V, Hazlerigg D. Maternal photoperiod programs hypothalamic thyroid status via the fetal pituitary gland. Proc Natl Acad Sci U S A. 2017 ;114(31):8408-8413. doi:10.1073/pnas.1702943114
Henningsen JB, Ancel C, Mikkelsen JD, Gauer F, Simonneaux V. Roles of RFRP-3 in the Daily and Seasonal Regulation of Reproductive Activity in Female Syrian Hamsters. Endocrinology. 2017 ;158(3):652-663. doi: 10.1210/en.2016-1689
Sáenz de Miera C, Monecke S, Bartzen-Sprauer J, Laran-Chich MP, Pévet P, Hazlerigg DG, Simonneaux V. A circannual clock drives expression of genes central for seasonal reproduction. Curr Biol. 2014; 24(13):1500-6. doi: 10.1016/j.cub.2014.05.024
Klosen P, Sébert ME, Rasri K, Laran-Chich MP, Simonneaux V. TSH restores a summer phenotype in photoinhibited mammals via the RF-amides RFRP3 and kisspeptin. FASEB J. 2013 ;27(7):2677-86. doi: 10.1096/fj.13-229559
Ancel C, Bentsen AH, Sébert ME, Tena-Sempere M, Mikkelsen JD, Simonneaux V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule. Endocrinology. 2012 Mar;153(3):1352-63. doi: 10.1210/en.2011-1622
Revel FG, Herwig A, Garidou ML, Dardente H, Menet JS, Masson-Pévet M, Simonneaux V, Saboureau M, Pévet P. The circadian clock stops ticking during deep hibernation in the European hamster. Proc Natl Acad Sci U S A. 2007 ;104(34):13816-20. doi: 10.1073/pnas.0704699104
Revel FG, Saboureau M, Masson-Pévet M, Pévet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr Biol. 2006 ;16(17):1730-5. doi: 10.1016/j.cub.2006.07.025